This event features two days of interactive workshops, presentations, panels and roundtables, all developed for smaller companies with limited resources.
Join us to gain a comprehensive education on clinical risk management and the tools to assess, maintain and mitigate risk to ensure compliance.
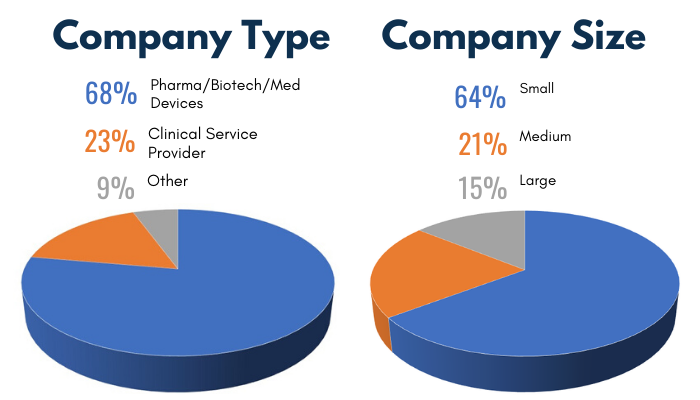
2019 Audience Demographics
TOP REASONS TO ATTEND
Having successfully established a series of clinical quality events on the East Coast and in Europe, ExL Events brought their well-known conference series to San Francisco in 2019, with a Clinical Trial Risk Management Seminar geared towards biotechnology and biopharmaceutical companies. With a successful first year under our belts, we are excited to come back and continue to provide the educational needs for smaller companies with limited resources.
- Understand the value of risk-based approaches to ensure compliance using limited resources.
- Ensure your clinical quality risk management plan covers all the critical elements to ensure GCP compliance.
- Develop an effective strategy to work with and oversee your CRO partners to ensure transparency, compliance and productivity.
- Learn the tools to achieve a constant state of inspection-readiness.
Who Should Attend
This conference is designed for professionals from pharmaceutical, biotechnology and medical device companies; CROs; and other clinical trial service providers whose responsibilities involve the following:
- Good Clinical Practice (GCP)
- Clinical Quality Assurance (CQA)
- Clinical Quality Control (CQC)
- Clinical Trial Operations/Management
- Clinical Research
- Quality Management/ Global Quality Management
- Audits/Inspections
- Compliance/Global Compliance
- Data Management/Systems Operations
- Clinical Monitoring
- Regulatory Affairs
- Safety and Risk Management/Operations
The event is also relevant to clinical QA, compliance and operations professionals from:
- Quality Service Providers and Consulting Companies
- CROs
- Central, Imaging and ECG Labs
- Investigative Sites
- IRBs
- Data Management and Software Vendors
- Safety Reporting Vendors